Charging Lithium-ion
Charging and discharging batteries is a chemical reaction, but Li-ion is claimed as an exception. Here, battery scientists talk about energies flowing in and out as part of ion movement between anode and cathode. This claim has merits, but if the scientists were totally right then the battery would live forever, and this is wishful thinking. The experts blame capacity fade on ions getting trapped. For simplicity, we consider aging a corrosion that affects all battery systems.
The Li‑ion charger is a voltage-limiting device that is similar to the lead acid system. The difference lies in a higher voltage per cell, tighter voltage tolerance and the absence of trickle or float charge at full charge. While lead acid offers some flexibility in terms of voltage cut‑off, manufacturers of Li‑ion cells are very strict on the correct setting because Li-ion cannot accept overcharge. The so-called miracle charger that promises to prolong battery life and methods that pump extra capacity into the cell do not exist here. Li-ion is a “clean” system and only takes what it can absorb. Anything extra causes stress.
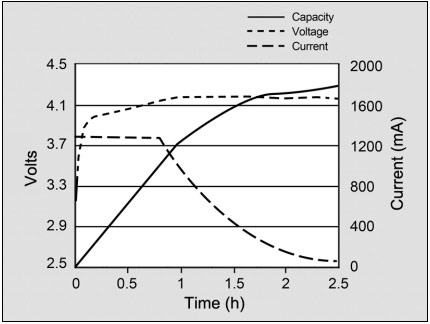
Most cells charge to 4.20V/cell with a tolerance of +/–50mV/cell. Higher voltages could increase the capacity, but the resulting cell oxidation would reduce service life. More important is the safety concern if charging beyond 4.20V/cell. Figure 1 shows the voltage and current signature as lithium-ion passes through the stages for constant current and topping charge.
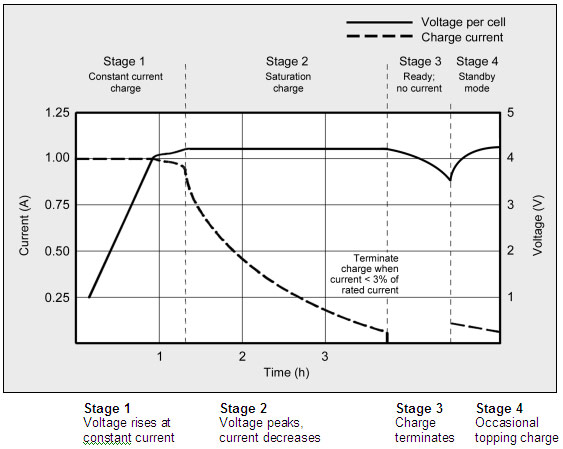
Figure 1: Charge stages of lithium-ion. Li-ion is fully charged when the current drops to a predetermined level or levels out at the end of Stage 2. In lieu of trickle charge, some chargers apply a topping charge when the voltage drops to 4.05V/cell (Stage 4).
Courtesy of Cadex
The charge rate of a typical consumer Li-ion battery is between 0.5 and 1C in Stage 1, and the charge time is about three hours. Manufacturers recommend charging the 18650 cell at 0.8C or less. Charge efficiency is 97 to 99 percent and the cell remains cool during charge. Some Li-ion packs may experience a temperature rise of about 5ºC (9ºF) when reaching full charge. This could be due to the protection circuit and/or elevated internal resistance. Full charge occurs when the battery reaches the voltage threshold and the current drops to three percent of the rated current. A battery is also considered fully charged if the current levels off and cannot go down further. Elevated self-discharge might be the cause of this condition.
Increasing the charge current does not hasten the full-charge state by much. Although the battery reaches the voltage peak quicker with a fast charge, the saturation charge will take longer accordingly. The amount of charge current applied simply alters the time required for each stage; Stage 1 will be shorter but the saturation Stage 2 will take longer. A high current charge will, however, quickly fill the battery to about 70 percent.
Li-ion does not need to be fully charged, as is the case with lead acid, nor is it desirable to do so. In fact, it is better not to fully charge, because high voltages stresses the battery. Choosing a lower voltage threshold, or eliminating the saturation charge altogether, prolongs battery life but this reduces the runtime. Since the consumer market promotes maximum runtime, these chargers go for maximum capacity rather than extended service life. Avoiding full charge has benefits, and some manufacturers set the charge threshold lower on purpose to prolong battery life. Table 2 illustrates the estimated capacities when charged to different voltage thresholds with and without saturation charge.
|
Charge V/cell |
Capacity at |
Charge time |
Capacity with full saturation |
|
3.80 3.90 4.00 4.10 4.20 |
60% 70% 75% 80% 85% |
120 min 135 min 150 min 165 min 180 min |
65% 76% 82% 87% 100% |
Table 2: Typical charge characteristics of lithium-ion. Adding full saturation at the set voltage boosts the capacity by about 10 percent but adds stress due to high voltage.
When the battery is first put on charge, the voltage shoots up quickly. This behavior can be compared to lifting a heavy weight with an elastic band. The lifting arm moves up quickly but the weight lags behind. The voltage of the charging battery will only catch up when the battery is almost fully charged (see Figure 3. This charge characteristic is typical of all batteries.
|
|
Figure 3: Capacity as a function of charge voltage on a lithium-ion battery The capacity trails the charge voltage, like lifting a heavy weight with an elastic band. Courtesy of Cadex
|
Relying on the closed circuit voltage (CCV) to read the available capacity during charge is impractical. The open circuit voltage (OCT) can, however, be used to predict state-of-charge after the battery has rested for a few hours. The rest period calms the agitated battery to regain equilibrium. Similar to all batteries, temperature affects the OCV. Read "How to Measure State-of-Charge".
Li-ion cannot absorb overcharge, and when fully charged the charge current must be cut off. A continuous trickle charge would cause plating of metallic lithium, and this could compromise safety. To minimize stress, keep the lithium-ion battery at the 4.20V/cell peak voltage as short a time as possible.
Once the charge is terminated, the battery voltage begins to drop, and this eases the voltage stress. Over time, the open-circuit voltage will settle to between 3.60 and 3.90V/cell. Note that a Li-ion battery that received a fully saturated charge will keep the higher voltage longer than one that was fast-charged and terminated at the voltage threshold without a saturation charge.
If a lithium-ion battery must be left in the charger for operational readiness, some chargers apply a brief topping charge to compensate for the small self-discharge the battery and its protective circuit consume. The charger may kick in when the open-circuit voltage drops to 4.05V/cell and turn off again at a high 4.20V/cell. Chargers made for operational readiness, or standby mode, often let the battery voltage drop to 4.00V/cell and recharge to only 4.05V/cell instead of the full 4.20V/cell. This reduces voltage-related stress and prolongs battery life.
Some portable devices sit in a charge cradle in the on position. The current drawn through the device is called the parasitic load and can distort the charge cycle. Battery manufacturers advise against parasitic load because it induces mini-cycles. The battery is continuously being discharged to 4.20V/cell and then charged by the device. The stress level on the battery is especially high because the cycles occur at the 4.20V/cell threshold.
A portable device must be turned off during charge. This allows the battery to reach the set threshold voltage unhindered, and enables terminating charge on low current. A parasitic load confuses the charger by depressing the battery voltage and preventing the current in the saturation stage to drop low. A battery may be fully charged, but the prevailing conditions prompt a continued charge. This causes undue battery stress and compromises safety.
Battery professionals agree that charging lithium-ion batteries is simpler and more straightforward than nickel-based systems. Besides meeting the voltage tolerances, the charge circuits are relatively simple. Limiting voltage and observing low current in triggering “ready” is easier than analyzing complex signatures that may change with age. Charge currents with Li-ion are less critical and can vary widely. Any charge will do, including energy from a renewable resource such as a solar panel or wind turbine. Charge absorption is very high and with a low and intermittent charge, charging simply takes a little longer without negatively affecting the battery. The absence of trickle charge further helps simplify the charger.
Overcharging Lithium-ion
Lithium-ion operates safely within the designated operating voltages; however, the battery becomes unstable if inadvertently charged to a higher than specified voltage. Prolonged charging above 4.30V forms plating of metallic lithium on the anode, while the cathode material becomes an oxidizing agent, loses stability and produces carbon dioxide (CO2). The cell pressure rises, and if charging is allowed to continue the current interrupt device (CID) responsible for cell safety disconnects the current at 1,380kPa (200psi).
Should the pressure rise further, a safety membrane bursts open at 3,450kPa (500psi) and the cell might eventually vent with flame. The thermal runaway moves lower when the battery is fully charged; for Li-cobalt this threshold is between 130–150C°C (266–302°F), nickel-manganese-cobalt (NMC) is 170–180°C (338–356°F), and manganese is 250°C (482°F). Li-phosphate enjoys similar and better temperature stabilities than manganese.
Lithium-ion is not the only battery that is a safety hazard if overcharged. Lead- and nickel-based batteries are also known to melt down and cause fire if improperly handled. Nickel-based batteries have also been recalled for safety concerns. Properly designed charging equipment is paramount for all battery systems.
Over-discharging Lithium-ion
Li-ion should never be discharged too low, and there are several safeguards to prevent this from happening. The equipment cuts off when the battery discharges to about 3.0V/cell, stopping the current flow. If the discharge continues to about 2.70V/cell or lower, the battery’s protection circuit puts the battery into a sleep mode. This renders the pack unserviceable and a recharge with most chargers is not possible. To prevent a battery from falling asleep, apply a partial charge before a long storage period.
Battery manufacturers ship batteries with a 40 percent charge. The low charge state reduces aging-related stress while allowing some self-discharge during storage. To minimize the current flow for the protection circuit before the battery is sold, advanced Li-ion packs feature a sleep mode that disables the protection circuit until activated by a brief charge or discharge. Once engaged, the battery remains operational and the on state can no longer be switched back to the standby mode.
Do not recharge lithium-ion if a cell has stayed at or below 1.5V for more than a week. Copper shunts may have formed inside the cells that can lead to a partial or total electrical short. If recharged, the cells might become unstable, causing excessive heat or showing other anomalies. Li-ion packs that have been under stress are more sensitive to mechanical abuse, such as vibration, dropping and exposure to heat.
Charging Lithium-ion Polymer
Charging Li‑ion polymer, also referred as Li-polymer, is very similar to a regular lithium-ion battery and no changes in algorithm are necessary. Most users won’t even know if their battery is Li‑ion or Li‑polymer. The word “polymer” has been used as promotional hype and does not reflect special attributes other than to know that the battery is built in a different way to a standard Li-ion.
Most polymer batteries are based on a hybrid architecture that is a cross between Li-ion and Li-polymer. There are many variations within the polymer family, and the true dry polymer battery for the consumer market is still years away. Also know as the “plastic battery,” this system was first announced in early 2000 but was never able to attain the conductivity needed for most applications at ambient temperatures. Read more about the Lithium-polymer battery and the Pouch Cell.
Simple Guidelines for Charging Lithium-based Batteries
-
A portable device should be turned off while charging. This allows the
battery to reach the threshold voltage unhindered and reflects the
correct saturation current responsible to terminate the charge. A
parasitic load confuses the charger.
-
Charge at a moderate temperature. Do not charge below freezing.
-
Lithium-ion does not need to be fully charged; a partial charge is better.
-
Chargers use different methods for “ready” indication. The light signal may not always indicate a full charge.
-
Discontinue using charger and/or battery if the battery gets excessively warm.
-
Before prolonged storage, apply some charge to bring the pack to about half charge.
-
Over-discharged batteries can be “boosted” to life again. Discard pack
if the voltage does not rise to a normal level within a minute while on
boost.